Abstract
Background
Acute lymphoblastic leukemia (ALL) in Down syndrome (DS) have uncommon genetic alterations such as mutations of JAK2, RAS, and overexpressions of CRLF2. These findings suggest DS-ALL may have unique biological features compared with non-DS-ALL. While recent studies implicated HMGN1 or DYRK1A in chromosome 21 were associated with molecular pathogenesis of DS-ALL, it remains to be elucidated what predispose DS children to develop ALL.
Materials and Methods
We performed whole transcriptome sequencing, targeted deep sequencing, and SNP array analysis in 25 DS-ALL samples, which included four ETV6-RUNX1 fusions and one high hyperdiploid. To compare with DS-ALL, we also performed whole transcriptome sequencing and whole exome sequencing to 118 non-DS-ALL samples, which included several subtypes such as ETV6-RUNX1 or BCR-ABL1. To cluster gene expression profiling, we applied the hierarchical clustering method. The detection of Ph-like signatures was performed by the hierarchical clustering by the gene set reported by Harvey.
Results
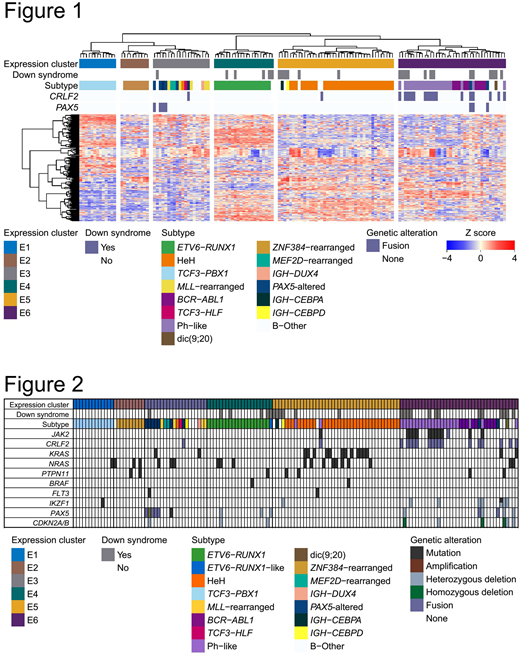
In expression analysis, we identified 19 fusions in 25 DS-ALL samples. These fusions included 15 recurrent fusions in pediatric BCP-ALL and 4 novel fusions, which including SSBP3-DHCR24, PDGFA-TTYH3, and NIN-NDUFA6. In novel fusions, PDGFA-TTYH3 fusions were detected in two DS-ALL samples. The hierarchical clustering analysis (Figure 1) combining 25 DS-ALL with 123 non-DS ALL samples. In our cohort, we defined samples with PAX5 alteration only such as a mutation or fusion as PAX5-altered. This clustering revealed ALL samples were divided into six clusters (cluster E1 to E6). Among six clusters, DS-ALL samples were divided into four clusters. In these four clusters, chi-square test revealed the significant enrichment of DS-ALL in E6 cluster. Importantly, our expression analysis revealed DS-ALL samples were highly heterogeneous and had the same expression pattern corresponding to each subtype same as non-DS-ALL. Cluster E3 included most samples with PAX5 fusions. All samples with ETV6-RUNX1 fusions were classified into cluster E4. Most samples of high hyperdiploid were classified into cluster E5. Cluster E6 was characterized by BCR-ABL1 fusions and Ph-like signatures. We detected 21 samples had Ph-like signatures, which included seven DS-ALL samples and 14 non-DS-ALL samples. Though we also analyzed differentially expressed genes between DS-ALL and non-DS-ALL, no genes on chromosome 21 such as HNGN1 or DYRK1A was significantly expressed. To investigate a relation between expression and genomic status, we further searched mutational analysis and copy number analysis (Figure 2). In 25 DS-ALL samples, six samples revealed JAK2 mutations and CRLF2 fusions. Interestingly, all of these six samples had Ph-like signatures. In cluster E5, one non-DS-ALL sample revealed JAK2 mutation and CRLF2 fusion and this particular sample was expected to have the Ph-like signature. To detect other Ph-like samples, we performed hierarchical clustering of 143 ALL samples based on the genes with a significantly (adjusted P value <0.0001) high expression in already detected 21 Ph-like samples. This analysis revealed three additional samples (two DS-ALL and one non-DS-ALL) had Ph-like signatures. Intriguingly, Ph-like samples accounted for 36% in 25 DS-ALL samples. In contrast, because several subtypes in non-DS-ALL showed mutations of RAS pathway genes, mutations of RAS pathway genes are common drivers in pediatric BCP-ALL. Copy number analysis elucidated one DS-ALL sample in cluster E3 had a known focal amplification of chromosome 9 involving exon 2 to 5 of PAX5, which may result in dysfunction of PAX5. Though no report analyzed PAX5 status except for deletion in DS-ALL, DS-ALL had not only deletion of PAX5, but also miscellaneous aberrations such as amplification or fusion. One DS-ALL sample without ETV6-RUNX1 in cluster E4 had homozygous deletions of ETV6, implicating ETV6-RUNX1-like signature.
Conclusion
Our result suggested DS-ALL were highly heterogeneous. Though expression profiles of DS-ALL had similar to non-DS-ALL, frequencies of subtypes in DS-ALL were quite different from non-DS-ALL, that is, low incidence of ETV6-RUNX1 or HeH, and high incidence of Ph-like signatures. Because molecular targeting agents such as imatinib or ruxolitinib improve the prognosis of Ph-like ALL, these agents may be also promising for treatment of DS-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal